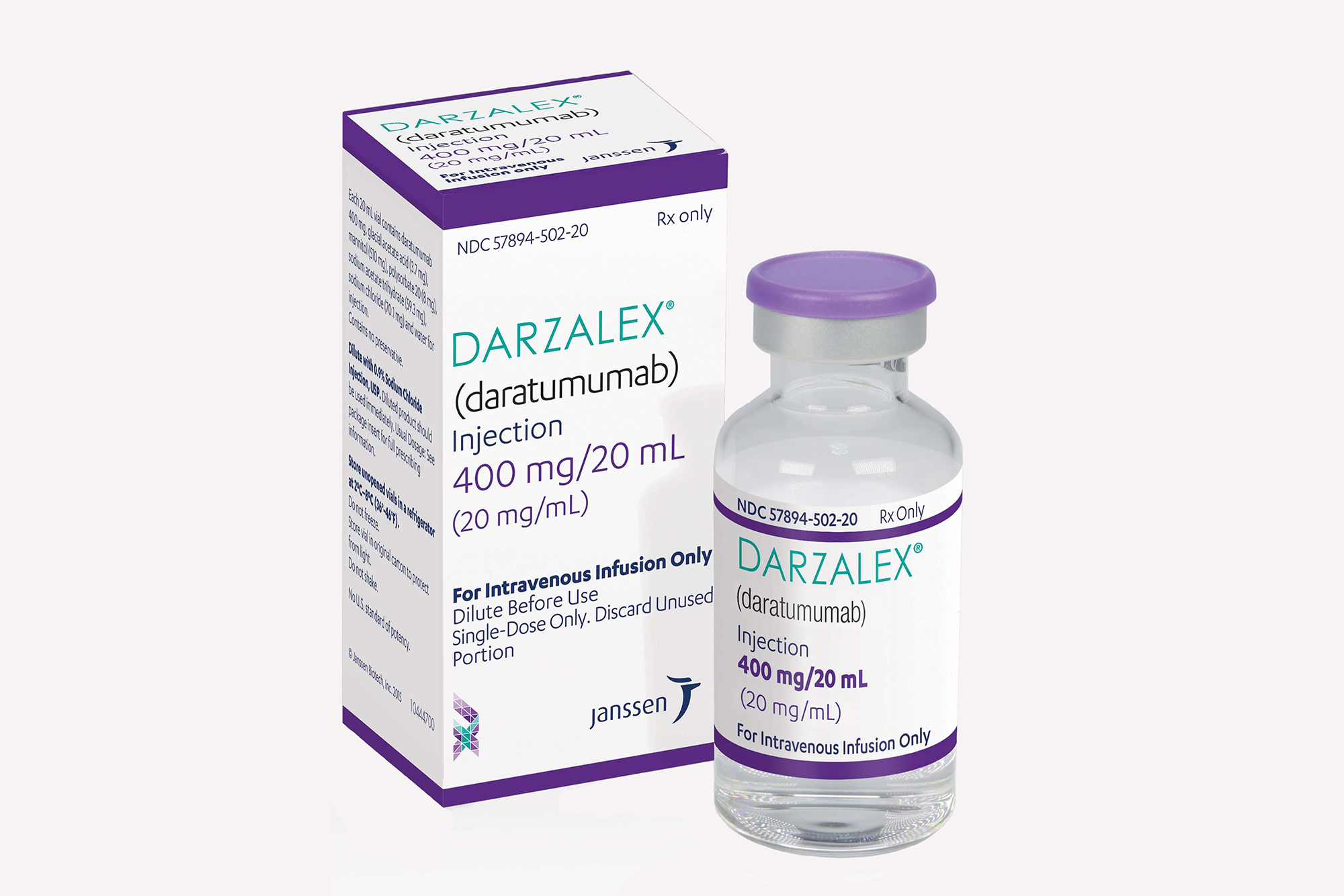
Darzalex 20 mg/mL intravenous solution
$483.81 – $1,906.69
Darzalex 20 mg/mL intravenous solution
Darzalex 20 mg/mL intravenous solution
(daratumumab) is used to treat multiple myeloma to help slow or stop cancer from progressing. Darzalex is a targeted cancer medicine that binds to a protein on the surface of myeloma cells, it then kills the cancer cells, and also helps the immune system to fight cancer better. It can be used alone as a single therapy but is often used together with other medicines, depending on your clinical situation. Darzalex is an infusion that is given into a vein over a period of hours by your healthcare provider. Darzalex subcutaneous form is called
Faspro which is given as an injection under the skin over 3 to 5 minutes. It is a different product as it contains both daratumumab and hyaluronidase-fihj. Darzalex FDA approval was granted on November 16, 2015, for multiple myeloma who had previously had other treatments. Darzalex infusion has been extended to treat a range of patients with different treatment regimes. Darzalex Faspro FDA approval was received on May 1, 2023
Before taking this medicine
You should not be treated with Darzalex if you are allergic to daratumumab.
To make sure this medicine is safe for you, tell your doctor if you have ever had:
- a breathing disorder; or
- herpes zoster (also called shingles) you may be prescribed antiviral medicine to prevent reactivation of shingles.
- hepatitis B as hepatitis B virus may become active again. Tell your healthcare provider right away if you get worsening tiredness or yellowing of your skin or the white part of your eyes.
- have hereditary fructose intolerance (HFI). This medicine contains sorbitol which is a source of fructose. People with HFI cannot break down fructose, which may cause serious side effects.
Dosage
Usual Adult Darzalex Dose for Multiple Myeloma
Dosing schedule in combination with lenalidomide or pomalidomide (4-week cycle) and low-dose dexamethasone and for monotherapy:
- 1 to 8 Weeks: 16 mg/kg IV weekly (total of 8 doses).
- 9 to 24 Weeks: 16 mg/kg IV every 2 weeks (total of 8 doses); first dose of the every-2-week dosing schedule is given at Week 9.
- 25 Week and onwards until disease progression: 16 mg/kg IV every 4 weeks; first dose of the every-4-week dosing schedule is given at Week 25.
Dosing schedule in combination with bortezomib, melphalan, and prednisone 6-week cycle:
- 1 to 6 Weeks: 16 mg/kg IV weekly (total of 6 doses).
- Weeks 7 to 54: 16 mg/kg IV every 3 weeks (total of 16 doses); first dose of the every-3-week dosing schedule is given at Week 7.
- 55 Weeks onward until disease progression: 16 mg/kg IV every 4 weeks; first dose of the every-4-week dosing schedule is given at Week 55.
| Quantity | 5 milliliters, 20 milliliters |
|---|
Be the first to review “Darzalex 20 mg/mL intravenous solution” Cancel reply
You must be logged in to post a review.
Related products
Medications
Medications
Medications
Medications
Medications
Medications
Medications
Medications





Reviews
There are no reviews yet.